Turbine Launches the World’s First Virtual Lab Using Cell Simulations
LONDON and BUDAPEST, HUNGARY – April 24, 2025 – In a landmark move, the FDA recently announced plans to phase out the requirement for animal testing in drug development, encouraging the use of human-relevant, in silico methods instead. This shift reflects a broader transformation in the life sciences, one that aligns closely with Turbine’s long-standing approach to drug discovery. In line with this evolution, Turbine is opening its previously internal virtual lab to the broader scientific community. The platform is being built on a decade of work developing a foundational cell model that allows scientists to simulate the outcome of experimental perturbations and gain insight into the molecular mechanisms that drive their results.
A First Step Toward a New Kind of Everyday for Scientists
This launch marks a turning point for Turbine: from a technology only accessible for partners in bespoke collaborations, to an open simulation-engine and virtual lab interface where scientists can design and iterate experiments at unprecedented speed and accuracy, across indications and cell types.
“For a decade, we’ve been developing our cell simulations hand in hand with select partners in biopharma R&D globally. The need to drive actionable insight that can be turned into an actual wet lab experiment or discovery decision was a forcing function on our technology,” said Szabolcs Nagy, Co-founder & CEO at Turbine. “When we realized that our biologists started rethinking the very process of biological experimentation by integrating simulations into processes, we knew we had to make this available to scientists everywhere. Over the past few years, we’ve built many simulated assays to predict and design experiments across R&D. We chose the ADC Payload Selector and Clinical Positioning Suite modules as the first impactful applications to open, but we intend to make all assay types available to partners in the coming months.”
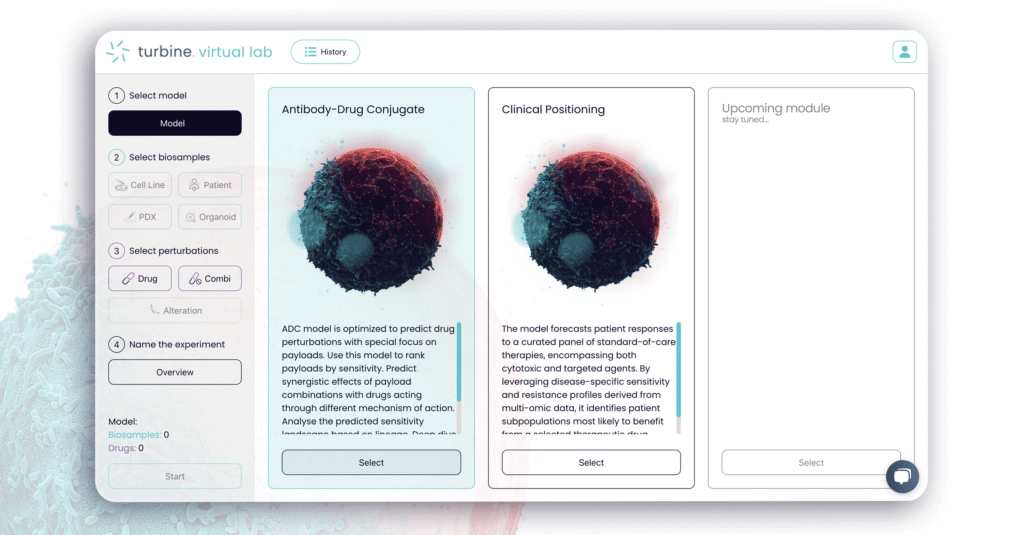
Now in Beta: the ADC Payload Selector and the Upcoming Clinical Positioning Suite
The first two beta modules being released in 2025 tackle some of drug development’s toughest questions, starting with antibody-drug conjugates (ADCs) and clinical positioning.
Simulations Are Key to Addressing a Major ADC Challenge
ADCs combine antibodies, linkers, and cytotoxic payloads – components that can be mixed in billions of ways. One of the biggest hurdles is payload-mediated resistance, where tumor cells adapt and lose sensitivity to treatment. Choosing the right payload is critical for efficacy and long-term response yet remains notoriously difficult to predict.
Turbine is aiming to address these and other ADC development challenges. Built on the company’s advanced cell simulations, the ADC Payload Selector beta allows researchers to:
- Identify and rank the most promising payload candidates by running millions of highly predictive simulations in an unbiased search space
- Understand context-specific effects with an in silico library of biological models that can fill data gaps
- Calculate combination synergy predictions at various doses and view detailed drug and cell model profiles
Building a Vast Virtual Patient Library, Starting with Champions Oncology
To ensure predictive accuracy in clinical positioning, Turbine is integrating clinically relevant tumor data from Champions Oncology. The collaboration enriches Turbine’s virtual patient library with multi-omics and real-world biological data mapped onto its foundational cell model, enabling researchers to simulate experiments on virtual patients built from harmonized real-world data. These simulations help predict therapeutic response across diverse molecular subtypes. As a result, researchers can now:
- Access a unique library of virtual patient models, capable of covering experimental perturbations otherwise unable to be investigated
- Simulate novel therapy responses across patients who never received treatment of the same nature
- Identify molecular traits linked to drug sensitivity or resistance to refine eligible patient cohorts for clinical trials
“The future of AI- and ML-driven drug discovery is grounded in access to precise, high-quality multi-omics datasets,” said Matt Newman, EVP at Champions Oncology. “Champions is uniquely positioned to meet this need, delivering the deeply characterized, clinically relevant data that partners like Turbine are seeking. Our dynamic, ever-evolving biobank – now enriched with cutting-edge modalities such as cell surface proteomics and genetic perturbation data – has the potential to fundamentally reshape oncology research and development.”
To request access to the beta modules, please visit www.turbine.ai/demo.
About Turbine
Over the past decade, Turbine’s interdisciplinary team of biologists and AI developers has built the world’s first interpretable cell simulation platform. Turbine’s technology addresses a critical challenge in biopharma R&D: despite an explosion of new ideas and technologies, it remains difficult to pinpoint which treatments will ultimately succeed in patients, resulting in over 90% of drug candidates failing in clinical trials and billions of dollars lost.
Turbine’s virtual lab enables in silico experimentation using a vast library of cell models, patient-derived samples and virtual patients. By computationally predicting therapy effects, drug developers can focus their resources and substantially increase the likelihood that new treatments will make it to the patients who need them the most.
Turbine’s simulations have been validated through partnerships with leading pharma and biotech companies, including Bayer, AstraZeneca, Ono, and Cancer Research Horizons, and the company continues to integrate its platform with other AI-driven discovery tools and CROs worldwide.
For more information, visit www.turbine.ai or follow our LinkedIn.
About Champions Oncology
Champions Oncology is a global preclinical and clinical research services provider that offers end-to-end oncology R&D solutions to biopharma organizations. With the largest and most annotated bank of clinically relevant patient-derived xenograft (PDX) and primary hematological malignancy models, Champions delivers innovative highest-quality data through proprietary in vivo and ex vivo platforms. Through its large portfolio of cutting-edge bioanalytical platforms, groundbreaking data platform and analytics, and scientific excellence, Champions enables the advancement of preclinical and clinical oncology drug discovery and development programs worldwide.
For more information, please visit www.ChampionsOncology.com.
back